Healthy Buildings Europe 2017
A packed room for one of the chemistry sessions.
An energetic talk by Prof. Miriam Diamond (University of Toronto).
Chemistry sessions
Jeff Siegel, University of Toronto “Indoor chemistry and building science: An opportunity for symbiosis”
Most (70-80 %) houses in North America have central forced air where the fan comes on as needed for conditioning purposes only. The operation of the fan in this system is rarely measured. There is a huge amount of variability in the operation of the fan within and between buildings. The operation of the fan can’t be predicted by temperature. Chemists need to understand and characterize this variation to understand indoor chemistry. At the same time, indoor chemistry can help understand buildings in new ways. Opportunity for symbiosis to advance building science and indoor chemistry agendas. Chemistry can help to better characterize the numerous surfaces in a building and whether those surfaces matter. Chemists can also help to identify new tracers that may better characterize exchange rate and allow us to characterize “unseen” spaces.
Nic Carslaw, University of York “Improved models for indoor air chemistry: Future needs and achievable goals”
In working towards the ultimate model framework, we must be able to predict across the vast and varied landscape of building environments. We cannot generalize (or predict) for individual buildings. The modelling workshop in Washington, DC (November 2016) provided a wishlist of measurements to improve predictive capabilities: Processes (gas-phase, aerosol-phase, surface interactions, photolysis), measurements (reactive species, secondary pollutants, photolysis). The newly-formed MOCCIE (Modelling Consortium for Chemistry of Indoor Environments) will work on indoor chemistry research questions from molecular to fluid dynamic in scale.
Drew Gentner, Yale University “Seeking a comprehensive chemical characterization of gas- and aerosol-phase organic compounds in indoor environments”
Chemical properties play an important role in determining partitioning. This has been well characterized outdoors, but does this translate indoors? Even under dry conditions, surfaces will be covered with a few monolayers of water. If all surfaces are covered in water, it’s possible that surface composition will be insignificant compared to the influence of water. Untargeted analysis with different ionization sources could be very useful in better understanding indoor chemistry. This technique can help to fingerprint and understand sources.
Jonathan Williams, Max Planck Institut für Chemie “Volatile organic compounds form people – an important indoor source”
Measurements of VOCs in areas where large numbers of people gather for events can help to understand human VOC emissions. Measurement at a soccer game with over 30,000 people in attendance indicate that emissions are low compared to large forests, but some distinct VOCs stand out, notably ethanol and acetonitrile. Similar measurements in a movie theatre during showing various films indicated that some VOC emissions can be predictably and reproducibly related to specific stimuli, particularly those that were classified as comedic or suspenseful.
Yingjun Liu, University of California Berkeley “Spatially and temporally resolved measurements of volatile organic compounds (VOCs) in a residence”
Using a PTR-MS were made at a home in Berkeley including from many distinct visible (e.g. living room, kitchen, etc.) and invisible (e.g. attic, crawl space, etc.) spaces as well as outdoors. VOC tracers showed which spaces were inter-connected or distinct. Emissions from specific activities, such as dishwasher use and making coffee, were clearly observed. Formic acid, acetaldehyde, acetone, ethanol, acetic acid, and methanol comprised up to 80 % of the total measured VOC signal in the living areas.
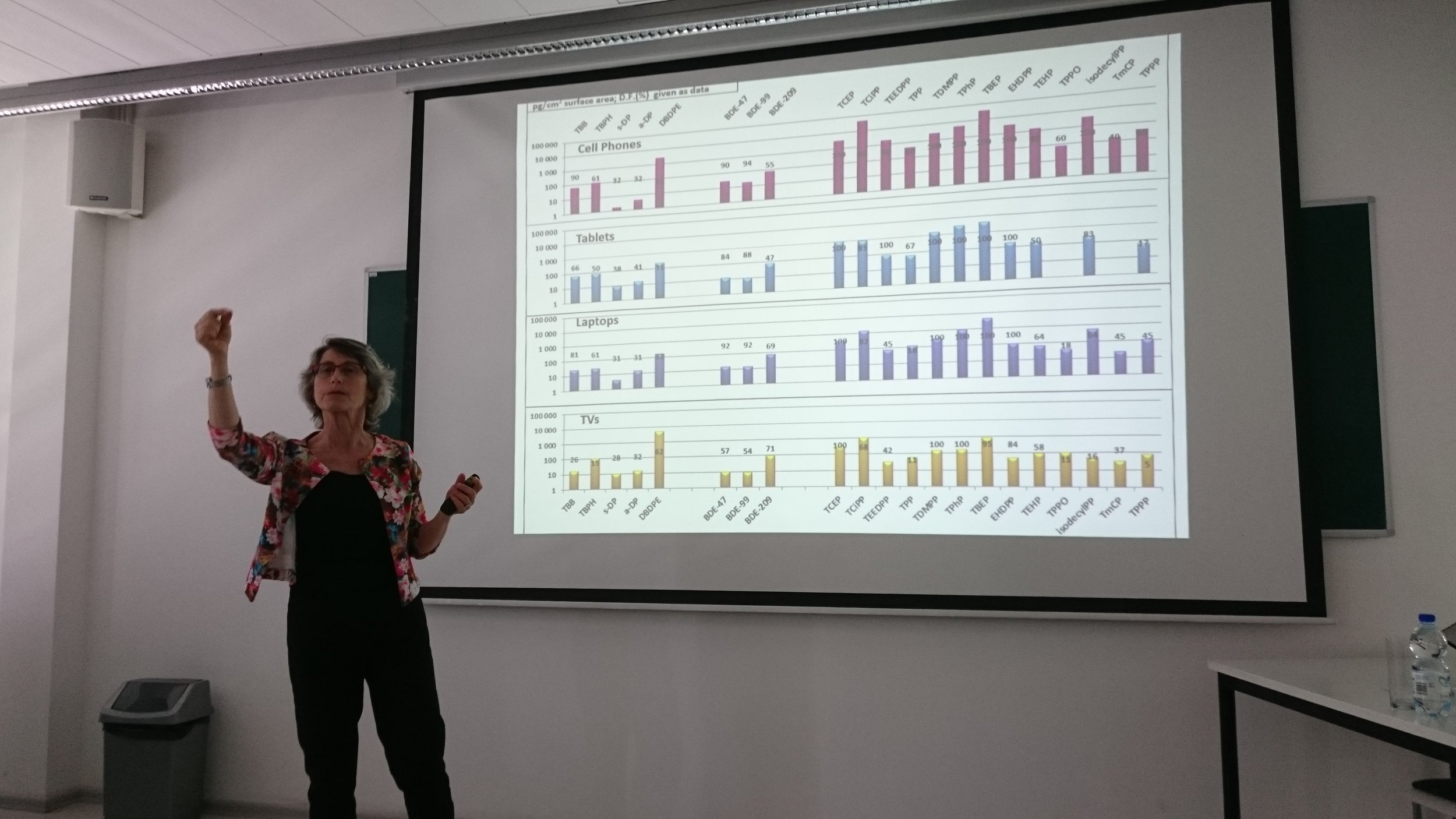
Miriam Diamond, University of Toronto “Organophosphates indoors – sources and sinks”
Flame retardants are known to be found in consumer products (e.g. electronics), which end up being found indoors at high concentrations. Individual items may comprise a dominant source to a given indoor environment, which can be tested using measurements and further evaluated using a model. A survey of legacy and replacement flame retardants on the surfaces of electronics found these compounds were ubiquitously present at high concentrations. This presents a risk for human exposure as use of electronics is increasing.
Tara Kahan, Syracuse University “Indoor photochemistry: Implications for HOx formation”
Most windows block light with wavelengths lower than 340 nm to the indoor environment. Other indoor light sources may be capable of initiating photolysis. Incandenscent, halogen, compact fluorescent, and fluorescent tube light sources all emit at wavelengths that could photolyse radical precursors. Light decays rapidly moving away from the light source but could be important for highly photolabile precursors found indoors, such as nitrous acid and hydrogen peroxide.
Jason Ham, National Institute for Occupational Safety and Health “Terpene oxidation indoors”
Understanding the reaction between ozone and terpenes is important because both are abundant in many indoor environments. Chamber studies show that NOx complicate the chemistry of the reaction. However, over-simplification of the reaction environment could lead to results that aren’t relevant. Development of a new method to effectively detect organic nitrates formed in these reactions could help to shed light on this chemistry.
Nadine Borduas, ETH Zurich “Tracking isocyanic acid (HNCO) in indoor environments”
Isocyanic acid is a toxic compound that is commonly present in the outdoor and indoor environment. Until recently, measurement techniques limited understanding of the chemistry of isocyanic acid. Using CIMS, the hydrolysis rate of isocyanic acid was determined under various conditions and was shown to be a minor loss process indoors. Study of the reaction of nicotine with hydroxyl radicals showed that it could isocyanic acid as well as other toxic products. Studies of isocyanic acid are undertaken in the occupational health and atmospheric chemistry communities separately. More communication between these complementary fields would be helpful.
Trevor VandenBoer, Memorial University “Measuring atmospheric alkyl amines in indoor environments”
Speciation and quantification of alkyl amines is important for the indoor environment, but analytical challenges have precluded the development of effective methods. Recent simple methods developed for remote outdoor environments could be applied to indoor environments. A pilot study deployed this method indoors and observed the presence of trimethyl amine and phenylamine, suggesting it could be further pursued to better understand the chemistry of these compounds indoors.
Overall themes from the chemistry sessions:
Lots of variability between indoor environments.
There is building variability that we aren’t yet considering (e.g. unseen spaces), where chemistry may be able to help.
Humans and their activities (e.g. breathing, cooking, cleaning, etc.) and products (e.g. phones, laptops, etc.) have a major impact on indoor spaces.
The chemistry that can happen indoors is poorly characterized compared to outdoors. Many differences between indoor and outdoor chemistry have been observed so far.
Chemicals that we haven’t yet considered may be important indoors.
Indoor chemistry is inherently interdisciplinary. Communication between disciplines can help to advance the field.
More measurements are needed!
Nadine Borduas (ETH Zurich) discusses organic nitrogen indoors.
Enjoying authentic Polish cuisine at the microbiome networking dinner.
Microbiome session
Jack Gilbert, The University of Chicago “Dead or alive: Microbial metabolism on built surfaces”
The effects of surfaces on microbes can be effectively studied using multi-omic analysis. When surfaces are wet, there is a decrease in taxonomic diversity and an increase in the number of individuals. These wet tiles become dominated by specific taxa faster than dry tiles. The location of the surface sets up the composition of the microbial community, but the moisture drives succession. Metabolism on different surfaces appears to reach a common endpoint.
Erica Hartmann, Northwestern University “The effect of antimicrobial chemicals on the built environment microbiome
Triclosan is a common antimicrobial used indoors that can lead to antibiotic resistance. Triclosan is found in dust, with levels varying between collection locations. Dust concentrations of triclosan weakly correlated with microbial community composition, but antibiotic resistance genes correlate strongly and significantly with triclosan concentration.
Anna Lawniczek-Walczyk, National Research Institute, Poland “Airborne transmission of microorganisms in occupational settings monitored using traditional biochemical and molecular methods”
Multiple samples collected from workers and workplace at power plants and analyzed using traditional and modern molecular methods. Biomass was shown to be the primary source of microbial exposure to workers, which can be easily transferred to the hands and masks of workers during their work activities. The dominant mechanism for environmental spreading of microbes was direct contact with biomass, followed by transmission through the air. Results could have implications for worker exposure.
Pawel Misztal, University of California Berkeley “Probing indoor microbial VOC emissions with PTR-ToF-MS”
Examining microbial VOC emissions in a normal building using coupon-collected biofilm purged into PTR-ToF-MS. Larger emissions were observed from the kitchen than the bathroom, with higher amounts in summer than winter. Correlation to gene copy density suggests the VOC emissions were microbial. Dodcanal, nonanal, and nonanoic acid appear to act as tracers for biofilm. These tracers were higher when people were present in the test environment. Microbial VOCs contribute low ppb levels to the total VOC loading indoors.
Overall themes from microbiome session
Surfaces and their properties are important to the microbiome.
Transmission of microbes through air is important.
Humans impact the microbiome through their presence and their use of products.
Biology and chemistry interact indoors. Both need to be considered!